Temporary urethral and ureteral stents coated with a silicone/polyurethane polymer to prevent encrustation and for easy removal
A silicone/polyurethane film coats the Nitinol structure of the Allium Stents, preventing the spring from becoming embedded. Removal is easy, even after several months of implantation.
Urethral stents (3) are indicated for the treatment of recurrent strictures of the bulbar urethra , sclerosis of the bladder neck , adenomas or strictures of the prostate for non-anesthesiable patients as well as for temporary incontinentation .
The URS ureteral stent is intended for ureteral strictures (tumor compression, radiation strictures, junction syndrome, ureteral fistula, iatrogenic post-ureteroscopy strictures, Bricker, etc.) instead of repeated placement of double J probes for long-term implantations (3 years).
These endoprostheses are all reimbursed by Health Insurance (LPPR code = 8112460).


TPS urethral stent for BPH or prostatic stenosis

RPS urethral stent for bladder neck sclerosis
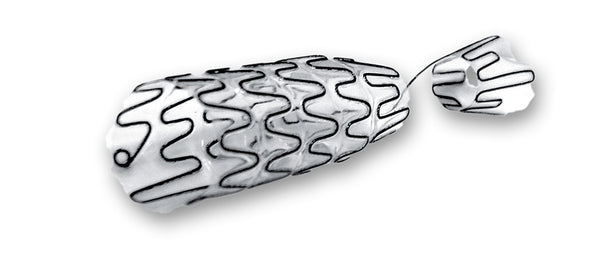
BUS urethral stent for anterior urethral stricture

URS stent for ureteral stricture